Niobium Mining
NIOBIUM :
The primary mineral from which niobium metal is extracted is known as pyrochlore. The world's largest deposit is located in Araxa, Brazil, and is owned by Companhia Brasileira de Metalurgia e Mineracao (CBMM). These reserves are estimated to be sufficient to meet the current global demand for approximately 500 years, totaling about 460 million tons. The extraction of weathered ore, containing between 2.5 and 3.0% Nb2O5, is carried out through simple open-pit mining, without the need for drilling and explosives. Roughly 85 to 90% of the niobium industry obtains its niobium ores from sources unrelated to the mining of tantalum-containing ores.
Another pyrochlore mine in Brazil is owned and operated by Anglo American Brazil Mineracao Catalao and contains 18 million tons at 1.34% niobium oxide. The third-largest pyrochlore deposit currently being actively mined is the Niobec Mine in Quebec, Canada, owned by Cambior, with reserves of 18,000 tons. In all three facilities, the pyrochlore mineral undergoes primarily physical processing techniques to produce a concentrate containing approximately 55 to 60% niobium oxide.
These three companies account for approximately 85% of the world's demand for niobium products, with much of that production taking the form of ferro-niobium, containing approximately 60% niobium oxide, used in the production of high-strength, low-alloy steel. Columbite, a mineral with a ratio of Nb2O5 to Ta2O5 ranging from 10:1 to 13:1, is found in Brazil, Nigeria, Australia, and other countries in central Africa. Niobium is recovered when ores are processed for tantalum. Additionally, niobium is found in very small quantities in the slag formed during the smelting of certain tin ores.
MINING AND PROCESSING :
Niobium ore is typically mined using the open-pit method, where it is divided into blocks and processed through ripping, bulldozing, loading, and transportation. This open-pit mining approach is employed in Brazilian regions, while underground methods are utilized in other areas containing niobium ore. Ore concentration is achieved through crushing and grinding, followed by magnetic separation to separate magnetite. Subsequently, desliming and flotation separation are conducted.
NIOBIUM ORES :
Niobium primarily occurs as an oxide and in conjunction with tantalum. Pyrochlore[(Na, Ca)2Nb2O6F] and columbite [(Fe,Mn)(Nb, Ta)2O6] are the principal mineral ores of niobium, comprising niobate, tantalate, iron, and manganese in specific proportions. Pyrochlore is typically found in carbonatites and pegmatites derived from alkaline rocks, often associated with zirconium, titanium, thorium, uranium, and rare-earth minerals. Columbite is commonly found in intrusive pegmatite, biotite, and alkaline granites. These ores are extracted as by-products of other metals due to their occurrence in small deposits and irregular distribution.
Significant pyrochlore mines are located in Brazil and Canada, while substantial columbite deposits are situated in Nigeria and Congo. In Nigeria, columbite concentrates are obtained as by-products of tin mining.
EXTRACTION AND REFINING :
Most commercial extraction and refining processes for the preparation of niobium consist of a series of progressive operations. After the mining process is carried out, the pyrochlore ore concentrates are reduced to ferroniobium, which is essentially niobium pentoxide. The procedure begins with the ore concentrates being mixed in a rotary mixer lined with magnesite refractory bricks along with hematite and aluminum powder. Following this, fluorspar and lime fluxes are added in small amounts before unloading the mixture into steel containers lined with magnesite refractory bricks. The charge is then placed in circular concave pits made from a mixture of lime, fluorspar, and silica sand. Reduction is induced by igniting a mixture of aluminum powder and sodium chlorate or barium peroxide. This exothermic reaction lasts for about 15 to 30 minutes, reaching temperatures of up to 2,400°C. The gangue impurities from the concentrate, including all thorium and uranium oxides, enter the molten slag. Once the entire reaction is complete, the slag is tapped off, and the vessel is lifted, allowing the metal to solidify in the sand, forming an alloy of ferroniobium.
This alloy is then crushed into particle sizes of 10 millimeters for marketing purposes. The content of this alloy is found to be 62 to 69 percent niobium, 29 to 30 percent iron, 2 percent silicon, and 1 to 3 percent aluminum. This ferroniobium is mixed with iron oxide and aluminum and is then reduced to obtain niobium and iron through the aluminothermic process. The component metals can be purified in an electron beam furnace, or the alloy can be used as is. Aluminothermic reactions are exothermic chemical reactions where aluminum acts as the reducing agent at high temperatures. These reactions are used in the production of several ferroalloys. In the case of reduction from ferroniobium to niobium, the reaction occurs as follows:
3 Nb2O5 + 10 Al --> 5 Al2O3 + 6 Nb
Ferroniobium is an important alloy for the production of niobium. 80% of the niobium production in the world is obtained through ferroniobium only.
TOP NIOBIUM PRODUCING COUNTRIES :
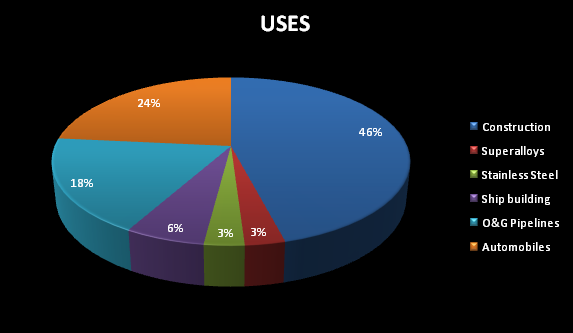
USES OF NIOBIUM :
It is used in the manufacturing of various types of steel.
Ferroniobium is alloyed with steel for marketing purposes.
Niobium oxide can be used in camera lenses and as a coating on glass for computer screens.
Niobium powder is useful in making niobium capacitors for electronic circuits.
Niobium sheets, wires, tubes, and rods are useful in physics experiments.
Niobium alloys are strong, which is why they are used in pipeline construction.
Other uses include making jet engines and rockets, beams and girders for buildings and oil rigs, and oil and gas pipelines.
ANNUAL NIOBIUM USAGE :
NIOBIUM PRODUCTION IN THE WORLD :
Canada is known as the second-largest producer of this rare metal, producing 4,167 metric tons per annum and contributing about 9% of the world's niobium production. North America relies solely on Canada for pyrochlore. The city with the largest deposit of niobium is Araxa, located in the Minas Gerais state, and also in Saint-Honore-de-Chicoutimi in Quebec, Canada. Brazil is the highest producer of niobium, currently producing 40,000 metric tons per year. Nigeria has reserves of 14,000 tons of niobium and is the second-highest African producer of niobium after Rwanda. Niobium mines are located in Nigeria in Jos Plateau, Kano, and Bauchi states. The mining industry in Nigeria contributes 0.3% to its Gross Domestic Product (GDP).
Related Mining