SILVER MINING
SILVER :
Silver is a soft, white lustrous transition metal that boasts the highest electrical conductivity of any element. Most silver is produced as a by-product of copper, gold, lead, and zinc refining. The metal occurs naturally in its pure form (native silver), as well as in alloys with gold and other metals, and in minerals such as argentite and chlorargyrite. This long-valued metal is not only used as an adornment; industrially, it finds application in electrical contacts and conductors, specialized mirrors, window coatings, and catalysis of chemical reactions. Compounds of silver are also utilized in photographic film and X-rays.
Generally, silver is extracted from ore through smelting or advanced chemical leaching techniques. Europeans discovered vast quantities of silver in the New World, notably in the present Mexican State of Zacatecas (exposed in 1546) and Potosi (Bolivia, also discovered in 1546), triggering a period of price increases in Europe.
The conquistador Francisco Pizarro was reported to have resorted to having his horses shod with silver horseshoes due to the abundance of the metal, in contrast to the scarcity of iron in Peru. Silver, highly valued in China, became a global commodity, contributing to the rise of the Spanish Empire. The fluctuation of its value significantly impacted the global market.
MINING AND PROCESSING :
Silver-bearing ores are mined using either open-pit or underground methods, after which they are crushed and ground. Despite the existence of other technologies, this metal is extracted through a process that employs gravity to break and extract its ores from large deposits. The extraction method for removing the ore varies depending on the physical characteristics of the rock surrounding the metal and the shape of the deposits containing the metal. These deposits are often long and cylindrical and are hence known as veins. The silver ores are robust and composed of solid substances, and they can also be found in sand, gravel, and other mineral deposits in the form of flakes.
Silver is often mined alongside the metal gold, found together in the form of an alloy called electrum. It is also regularly mined alongside other elements, including argentite, pyrargyrite, and cerargyrite, resulting in the end product known as Horn Silver. Silver mostly occurs as a secondary element along with lead, copper, and zinc ores, which is why nearly 50 percent of the silver mined today is obtained during the processing of other types of ore. It is separated from other ores through the smelting process.
When a new silver mine is developed, a system of multi-layered crosscuts is constructed, where each cut connects to a central shaft but is maintained at a safe vertical distance to prevent collapses. Openings called raises are dug to connect each level, dividing the ore body into blocks. At this stage, the silver mine is ready for extraction. The overhand stoping method is often employed, where the ore is removed starting from the bottom and working up one layer at a time. Typically, mines comprise a network of tunnels and chambers designed to safely transport the pulverized ore up and out in mine cars.The oldest silver mines still in operation are located in Peru and Norway.
SILVER ORES :
Although certain silver-bearing ores contain silver, none of them contain silver as their major constituent. For instance, a particular ore might contain 0.085 percent silver, 0.5 percent lead, 0.5 percent copper, and 0.3 percent antimony. After flotation separation, the concentrate would contain 1.7 percent silver, 10 to 15 percent lead, 10 to 15 percent copper, and 6 percent antimony. It is known that approximately 25 percent of the silver produced comes from ores mined specifically for their silver content, while the remaining 75 percent comes from ores where silver is a major metal value, such as lead, copper, or zinc. These mineral ores are generally sulfides, with lead present as galena (PbS), zinc as sphalerite (ZnS), and copper as chalcopyrite (CuFeS2). Silver mineralization typically includes significant amounts of pyrite (FeS2) and arsenopyrite (FeAsS). The major silver ores are considered to be argentite (Ag2S), proustite (Ag3AsS3), and polybasite [(Ag,Cu)16Sb2S11]. More than half of the world's reserve base of silver mineralization is held by the United States, Canada, Mexico, Peru, Kazakhstan, and Russia.
EXTRACTING AND REFINING :
The metallurgical processes applied to a silver-bearing mineral concentrate depend on whether the major metal is copper, zinc, or lead. In most cases, the ore is mined and then treated by either mechanical or gravitational means to concentrate the ore minerals and separate them from the mass of non-ore material.
The primary ore of silver is argentite, and silver is extracted from argentite through the hydrometallurgy process. Firstly, the silver ore is dissolved in a cyanide solution to produce a soluble argentocyanide complex, from which the metal can be obtained through the reduction method. The different steps involved in the extraction of silver are as follows:
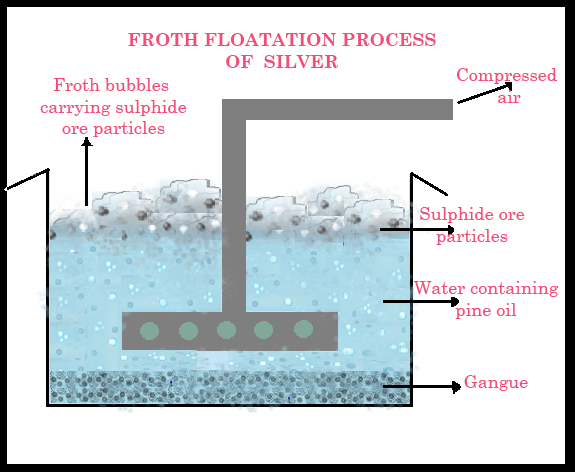
1. Ore- Concentration :
The ore Argentite is a sulfide ore and is thus concentrated by the froth flotation process. After the ore is crushed and reduced to fine particles, it is placed in a large tank containing water and pine oil as ingredients. This mixture is then agitated by passing compressed air through it, causing the ore to form a froth with the pine oil and rise to the surface, while the other impurities remain in the water below.
2. Treatment with sodium cyanide :
The concentrated ore obtained from the above process is treated with a 0.4% to 0.7% aqueous solution of sodium cyanide, and a current of air is passed through it. As a result, the argentite ore dissolves in the sodium cyanide solution to form Sodium Argento Cyanide. The reaction is as follows:
Ag2S + 2NaCN ------ > 2Na [Ag(CN)2] + NO2S
The reaction is a reversible one and so, oxygen is passed to oxidize Na2S to Na2SO4 such that the equilibrium shifts towards the product.
Na2S + O2 ------ > Na2SO4
The entire solution is then filtered, and the filtrate containing Sodium Argento Cyanide undergoes precipitation to recover the silver metal.
3. Precipitation of Silver :
The filtered solution of Sodium Argento Cyanide obtained is treated with zinc scrap, where zinc displaces silver from its complex. Thus, Sodium Zinc Cyanide is produced, and silver precipitates.
Zn + 2Na [Ag (CN)2] ----- > NO2[Zn (CN)4] + 2Ag
This silver precipitate is then collected, washed and fused to get a compact mass of silver.
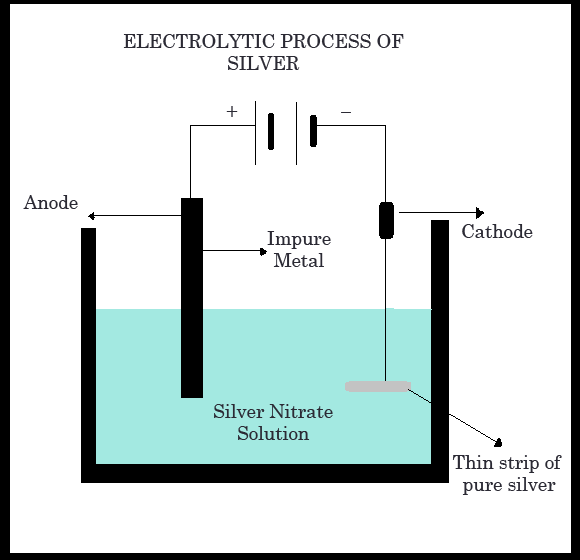
4. Refining :
The ore containing silver needs to be refined to obtain pure silver as the end product. The silver precipitate obtained previously may contain some impurities, so the impure silver is purified by the electrolytic method. The process involves placing a block of impure metal as the anode, while a thin strip of pure silver serves as the cathode. A mixture of silver nitrate solution is used as the electrolyte. When current is passed through the electrolytic tank, the impure silver dissolves, and an equivalent amount of pure silver is deposited at the cathode.
AgNO3(aq) ----- > Ag+ + NO3
At cathode: Ag+ + e ----- > Ag
At anode: Ag ----- > Ag+ +e
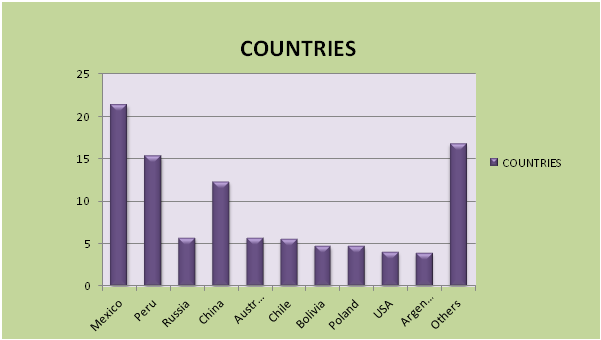
TOP SILVER PRODUCING COUNTRIES :
1. China: China currently stands as the world's number one silver producer, having produced 4,300 metric tons in 2019. The nation has long been a major player in the silver market, and its production has steadily increased in recent years. Shandong, Inner Mongolia, and Henan are among China's top silver-producing provinces.
2. Mexico: Mexico ranks as the second-largest silver producer globally, with a production of 3,700 metric tons in 2019. The country boasts a rich history of silver mining, and its production has steadily risen since 2015. Zacatecas, Chihuahua, and Durango are the leading silver-producing states in Mexico.
3. Peru: Peru holds the position of the third-largest silver producer worldwide, having produced 2,900 metric tons in 2019. The country has a long-standing tradition of silver mining, and its production has been consistently increasing since 2014. Arequipa, Puno, and Cajamarca are the top silver-producing provinces in Peru.
4. Russia:Russia is the fourth-largest silver producer globally, with a production of 1,800 metric tons in 2019. The country boasts a rich history of silver mining, and its production has steadily increased since 2011. Sverdlovsk and Orenburg are the leading silver-producing regions in Russia.
5. Australia: Australia ranks as the fifth-largest silver producer in the world, having produced 1,700 metric tons in 2019. The country has a lengthy history of silver mining, and its production has steadily risen since 2012. New South Wales and Western Australia are the top silver-producing states in Australia.
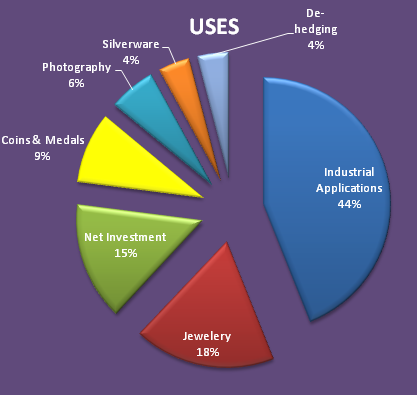
USES OF SILVER :
It is used in making ornaments, coins, and decorative articles, among other things.
It is utilized in the electroplating of silver and in making silver mirrors, among other applications.
Silver halides are employed in conventional x-ray film as an image receptor.
Silver sulfadiazine, an anti-infective medicine, is used as a topical cream to treat burns.
Silver is incorporated into bandages and dressings to prevent the spread of infections, as well as into gels and ointments for minor scrapes and cuts.
Silver nitrate has long been used as drops for newborn babies to prevent pink eye.
Colloidal silver is applied to treat ceramic water filters, aiding in the eradication of bacteria and pathogens, and facilitating the provision of clean water to developing nations.
Silver Coat technology is utilized in Foley catheters in urology to prevent urinary tract infections resulting from catheter use.
ANNUAL SILVER USAGE :
The annual silver usage rate can vary significantly depending on the type and purpose of its usage. Silver finds application in a variety of industrial, medical, and decorative contexts.
Industrial: Silver serves as a catalyst in industrial processes, including the production of chemicals, pharmaceuticals, and electronics. It is also utilized in plating, soldering, and electrical equipment. According to the International Silver Trade Association, the annual silver usage rate in industry was approximately 1,500 tons in 2017.
Medical: Silver is employed in medical applications like medical implants and wound dressings due to its antimicrobial properties. The World Silver Survey reports that the annual silver usage rate for medical applications was around 350 tons in 2017.
Decorative: Silver is used in decorative applications such as jewelry and tableware. The World Silver Survey indicates that the annual silver usage rate for decorative purposes was about 1,000 tons in 2017.
SILVER PRODUCTION IN THE WORLD :
Countries like Peru, Poland, Norway, Canada, and the U.S. are world leaders in silver mining, with Mexico serving as the country with the largest annual silver production. Silver is also mined in Bolivia. In Europe, all the silver mined is known to be extracted in the form of lead sulfide ore, also known as Galena. In Australia, the Cannington mine is one of the world's largest mines in terms of silver reserves. A very few mines in North America extract silver alone, while the U.S. primarily mines for zinc, lead, and copper. The United States leads in silver mining, particularly in Arizona, Montana, Nevada, and Idaho. The top five countries are as follows:
|
|
(tonnes) |
|
Related Mining