Zirconium Mining
ZIRCONIUM:
Zirconium is a metallic chemical element found in both the land and water of the Earth. Pure zirconium metal is used in nuclear reactors.
ORE:

The principal ore of zirconium is zircon. Zircon is found as a byproduct of titanium ores such as ilmenite and rutile. It can also be found in alluvial deposits and beaches, which are now the largest contributors of zircon for commercial production. Zircon consists of zirconium in the form of zirconium silicate (ZrSiO4).
MINING:

The zircon, as a byproduct of ilmenite and rutile, is mined through open-pit mining. The initial process of open-pit mining involves checking the surface, assessing ore quality and distribution, and evaluating the surrounding area. Once the necessary requirements are confirmed based on these factors, the mine is established. Mines are excavated as stepped walls with benches and ramps for transportation of miners, machinery, goods trucks, and waste.
The mines are often excavated in a circular pattern for deeper excavation. Miners handle drilling and blasting to extract ore from the mines. The zircon-containing ores excavated are collected and transported by trucks to nearby processing plants, which are typically set up near the mines. The collected ilmenite and rutile ores are usually heavier and larger in size. Open-pit mining has the advantage of being less expensive, easily shut down, and allowing unrestricted machinery usage, which is why it is widely employed.
Zircons found in alluvial deposits and beach sands are mined using dry mining methods with excavators and scrapers. Scrapers remove surface dirt from the deposits and collect zircon ores, while excavators dig out zircon ores, which are then piled in a designated area. The collected zircon ores are transported to nearby processing plants by bulldozers or trucks.
Dredge mining is employed in areas where zircon ore deposits are below the water table. In dredge mining, zircon ores are scooped from the water body by continuous bucket railing from the surface. The buckets stockpile the zircon sands and transport them to the initial processing plant, where zircon is separated, leaving tailings that are returned to the water body and refilled using the same bucket railing method.
processing and extraction:
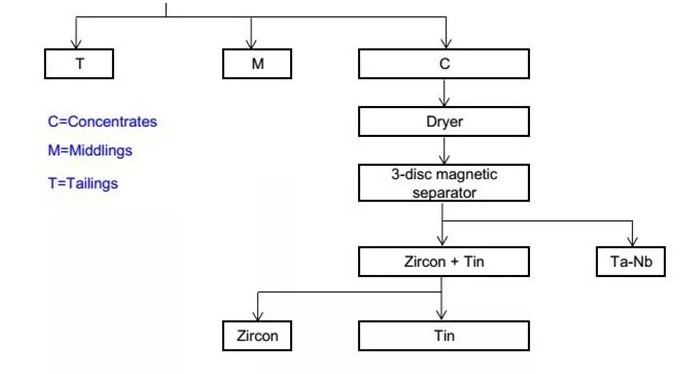
The zircon-containing ores from open-pit mining are milled and crushed. Zircon is separated from other ore particles using the magnetic separation method. In this method, metallic ores and non-metallic ores are separated based on their magnetic properties, with zircon being collected as a metallic ore, separating itself from non-metallic impurities like quartz, clay, and dirt.
Zircon sands from alluvial deposits and beaches are directly subjected to mechanical separation to isolate zircon from quartz and clay tailings. The mechanical separation method separates components based on the weight and density of the particles.
Zircon from wet deposits is separated using wet spiral separators. In these separators, separation is based on the weight and speed of the sliding materials.
Most zirconium is commercially used as zircon, with further polishing and refining. Only a very small amount is used as metal in nuclear reactors. The top zirconium-producing nations are Australia, South Africa, China, and Indonesia, along with Mozambique, India, and Sri Lanka. Australia and South Africa jointly account for 65% of world production.
DEFINITION:
Zirconium is a chemical element with the symbol Zr. It is a shiny, gray-white, strong transition metal that resembles titanium. Zirconium is used as an alloying agent due to its high resistance to corrosion. It is never found as a native metal; it is obtained mainly from the mineral zircon, which can be purified by chlorine.
PROPERTIES:
Zirconium is a shiny, grayish-white, soft, ductile metal that is solid at room temperature, though it becomes hard and brittle at lower purities. In powder form, zirconium is highly combustible, but the solid form is far less prone to igniting. Zirconium is highly resistant to corrosion by alkalis, acids, saltwater, and other agents.
APPLICATION:
Due to zirconium's outstanding resistance to corrosion, it is frequently used as an alloying agent in materials exposed to corrosive agents, such as surgical appliances, explosive primers, vacuum tube getters, and filaments. Zirconium dioxide (ZrO2) is used in laboratory crucibles, metallurgical furnaces, and as a refractory material. Zircon (ZrSiO4) is cut into gemstones for use in jewelry. Zirconium carbonate (ZrCO3·xH2O) was used in lotions to treat poison ivy, but this was discontinued as it caused bad skin reactions in some cases.
Related Mining

















